OVERVIEW
 Now that students have some background as to why mercury is an issue for humans and the environment, they need to begin to get some idea about where it comes from and how it develops into a toxic form that can be ingested by living things.
Now that students have some background as to why mercury is an issue for humans and the environment, they need to begin to get some idea about where it comes from and how it develops into a toxic form that can be ingested by living things.
Students need to develop a basic understanding of how mercury gets into their pond, field, forest, stream, or other area that they are studying. They also need to have a general feel for the factors that need to be present in order for the mercury to change into a form that can be absorbed by living things. In short, the students need a basic understanding of mercury chemistry.
There's more to it than the mercury chemistry, of course. Students also need to understand how bioaccumulation works, which means understanding the operation of food chains and food webs. We will focus on food chains and food webs in Unit 2. Here, in this first unit, our objective is just to introduce the following basic ideas:
- Mercury is toxic in very small quantities.
- Mercury occurs in a number of forms in nature. One of these forms- methylmercury- can be absorbed by living things and is very toxic.
- In the Northeast, most mercury arrives through the air.
- Methylmercury is the byproduct of metabolism by anaerobic bacteria, typically living in an acidic environment with access to carbon and sulfur.
Before engaging in Road to Toxicity you will probably want to review the mercury cycle yourself. has provided this schematic:
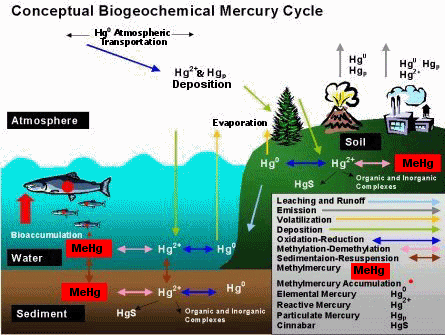
Additionally, the offers a simple diagram (if using this diagram you may want to explain that the “organic mercury” is a reference to methylmercury).
The has reprinted a useful schematic of the aquatic mercury cycle.
The Northeast Waste Management Association has published .
If you are looking for a description of the cycle that digs more deeply in to the chemistry (probably for your own use more than for students), is very useful.
About this Activity
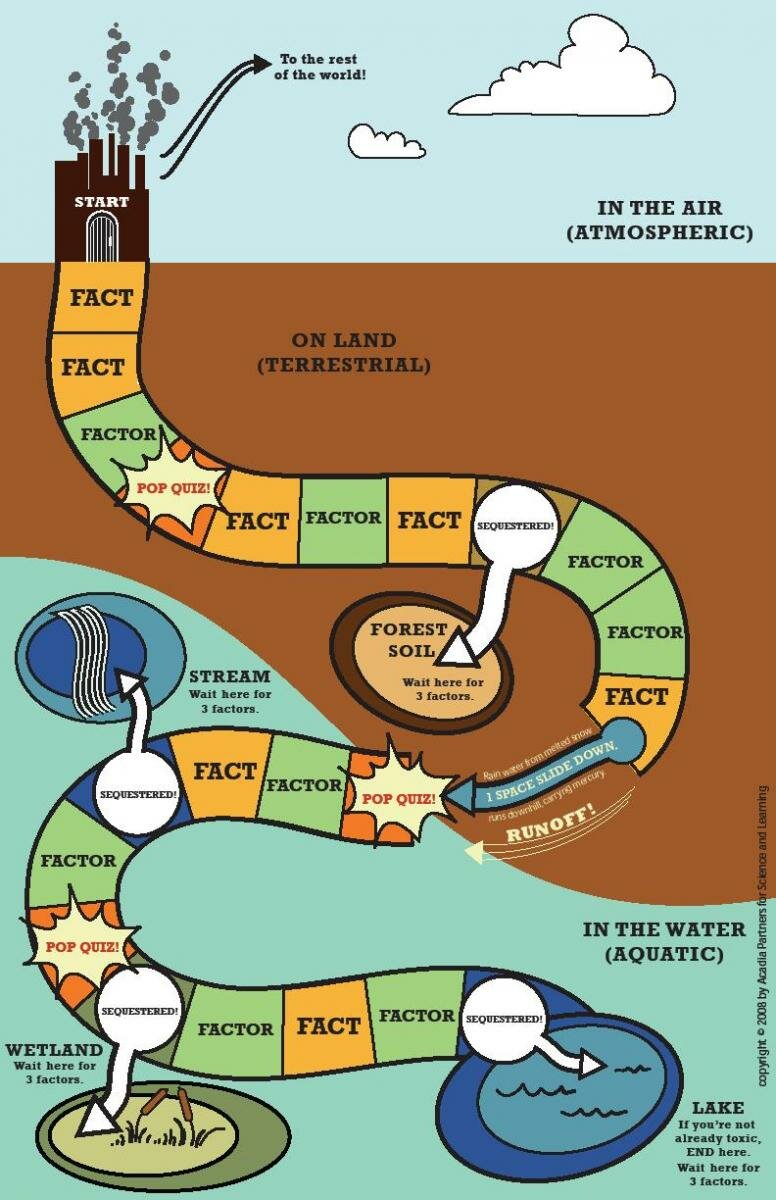
This activity is built around a board game in which the students compete to become “toxic.” Each student starts out as airborne mercury*- the kind of mercury that is captured by the needles and leaves of the trees in the watersheds that the students are studying. The goal is to convert to methylmercury. To do that, they need to bring together the three critical factors required for the transformation: the right chemistry (mercury, sulfur, and organic carbon, preferably in an acidic environment), an anaerobic environment, and the sulfate reducing bacteria that take inorganic mercury and convert it to methylmercury as a byproduct of metabolism.
Students can acquire these three critical factors either by landing on the right spaces and picking up a card—or by “stealing” the required factor from another student who is not able to answer a “pop quiz” question about mercury.
As students move around the board, they also pick up the “facts” about mercury that the need to provide correct answers on the pop quiz.
GETTING READY
Materials
Print out enough copies of the game board (on card stock if available) and the rules, cards, and game pieces so that students can work in groups of 3 or 4 as they play the game. Students can cut out cards and pieces. You will need a single six sided dice for each group. Here are the files that you need:
- Game Board (choose a size):
- Sized to print on a single 11x17 sheet of paper, all-in-one
- Sized to print on a pair of 8.5 x 11 sheets of paper, top half & bottom half
- Pieces
- Rules and fact and factor cards
The above files are also available for download from the Lesson Resources page.
Student Prerequisites
- How to measure volume and square inches
- Parts of a circle
- Proportions
- Averages
- Ranges
- Computer skills
- Library skills
ACTIVITY PROCEDURE
Doing the Activity
Using the game will be more successful for most students if you provide them with some background about the mercury cycle before introducing the game. It is probably useful to put this background information in the context of the field work that the students will be doing, in addition to relating it to the game. You might want to print off one of the mercury cycle diagrams referenced above and then take the students through the different phases of the cycle.
Working in groups, students can play the Road to Toxicity game until you feel assured that most of them have developed mastery of the key facts about mercury chemistry and how it moves from inorganic into an organic, toxic form. Once game play is complete, you will probably want to revisit the mercury cycle once again with the class as whole, using the assessment questions, below to check and correct understanding.
The Rules
The goal of the game is to become TOXIC - to be converted from inorganic mercury to methylmercury, the form that bioaccumulates in organisms. You can become toxic in soils, lakes, streams, or wetlands - depending where you land.
- Each player picks a game piece (thermometer, power plant, volcano, hazardous waste).
- Shuffle each stack of cards (FACT and FACTOR) and place in a central location.
- Take turns rolling 1 die and move the number of spaces indicated.
- If you land on a silver space, pick up a FACT card. Read the card aloud to the other players - the information will be important later. Keep the card.
- If you land on a green space, pick up a FACTOR card. Read it aloud to the other players. Keep this card
*Scientists are currently debating the amount of each form in the atmosphere, how each moves, and how long they stay aloft. The three forms are usually called: Hg0 (gaseous elemental mercury, GEM), Hg2+ (reactive gaseous mercury, RGM), and Hgp (particulate-bound mercury) (Lindberg et al. 2008).

 Acadia Learning brings scientists, teachers, and students together in partnerships that result in useful research and effective science education.
Acadia Learning brings scientists, teachers, and students together in partnerships that result in useful research and effective science education.